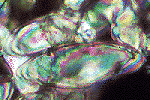![]() Acicular crystals. In other
cases the crystallization produces acicular crystals (from 2 µm
in diameter and up to 30 µm long) of calcite, called whiskers. They occur inside discrete pores (channels, vughs), forming
pseudomycelia, build up of a network of more or less interwoven crystals
inside the voids. They are found preferably in the upper parts of calcitic horizons
and represent a very typical case of the crystallization of pedogenic carbonates.
They form a first phase of precipitation of carbonates in the soil and are
supposed to be formed at the expense of oversaturated solutions.
Acicular crystals. In other
cases the crystallization produces acicular crystals (from 2 µm
in diameter and up to 30 µm long) of calcite, called whiskers. They occur inside discrete pores (channels, vughs), forming
pseudomycelia, build up of a network of more or less interwoven crystals
inside the voids. They are found preferably in the upper parts of calcitic horizons
and represent a very typical case of the crystallization of pedogenic carbonates.
They form a first phase of precipitation of carbonates in the soil and are
supposed to be formed at the expense of oversaturated solutions.